Abstract
Background
To recognize the growing list of recurrent genetically defined eosinophilia driven by constitutively active tyrosine kinase fusion genes, the World Health Organization (WHO) created a provisional entity of myeloid/lymphoid neoplasms with eosinophilia (MLN-Eo) with rearrangement of PFGFRA, PDGFRB or FGFR1, or with PCM1- JAK2. These eosinophilic disorders are clinically and genetically heterogeneous. Annotating additional somatic mutations and relevant clinical features may have an impact on treatment selection and refine prognostication. In this study, we aim to describe the clinicopathologic characteristics and outcome with upfront targeted tyrosine kinase inhibitor (TKI) therapy in patients (pts) with MLN-Eo.
Methods
We retrospectively reviewed clinical and molecular data on 39 pts with newly diagnosed MLN-Eo with PDGFRA, PDGFRB, FGFR1, JAK2 or FLT3 rearrangement at Moffitt Cancer Center, Duke Cancer Center, and the UCSF Comprehensive Cancer Center. Clinical data was abstracted in accordance with institutional review board approved protocol. Pts were divided to two categories based on morphologic classification of the disease at diagnosis or at transformation: chronic phase and blastic phase disease. Each category was then divided into two subgroups: Cohort A) upfront TKI +/- other systemic therapy and Cohort B) no upfront TKI arm. Median time from chronic phase disease to blastic phase and median overall survival (mOS) (in whole cohort) were calculated using Kaplan Meier method and compared with log-rank test. Cox proportional hazards (PH) model was used to calculate hazard ratio (HR) in univariate analyses.
Result
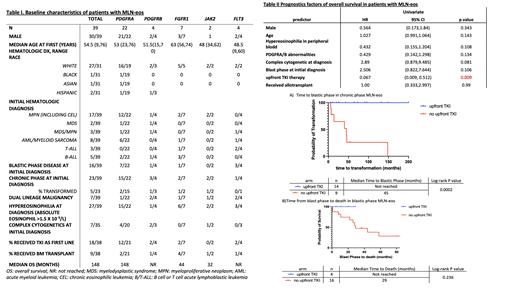
Among the 39 pts included in the analysis, 22 pts had PDGFRA fusion (20)/activating mutations (2), 4 had PDGFRB, 7 had FGFR1, 2 had JAK2 (PCM1-JAK2 and BCR-JAK2), and 4 had FLT3 (3 ETV6-FLT3 and 1 FLT-3-TRIP11). Median age at first diagnosis of myeloid/lymphoid neoplasm was 54.5 years (range 9-76). Seventy-seven percents (30/39) were male. Chronic eosinophilic leukemia (CEL) was the most common clinical diagnosis and occurred in 11 pts (28%). Seven (18%) pts had both myeloid and lymphoid neoplasms either concurrently or sequentially. Sixteen (41%) pts presented with de-novo blastic phase at time of initial diagnosis.
Among 23 pts who presented with chronic phase disease at diagnosis, nine patients did not receive TKI upfront. Five out of the nine patients (55%) developed blastic phase disease with a median follow up of 73 months. The median time to blastic phase was 45 months. No patients in the upfront TKI arm (n=14) had blastic transformation during follow up (Figure 1a, p<0.001).
Among 21 pts who had blastic phase disease (16 pts with de-novo diagnosis and 5 pts with blastic transformations), 95% of them (20/21 pts) had treatment information and follow-up data available. At a median follow up of 37 months, 11 pts (55%) were deceased, and median OS was 44 months. Seven pts (35%) underwent allogeneic stem cell transplant (alloHSCT). Four patients received upfront TKI, and all of them achieved complete remission and were alive at the time of the study (Figure 1b). Among those pts, 2 had FLT3-ETV6 rearrangement, 1 with PDGFRA rearrangement and 1 with FGFR1 rearrangement. Two pts received single agent TKI only and two pts received TKIs followed by alloHSCT.
In the univariate analysis, upfront TKI use was significantly associated with improved OS (HR 0.067, 95% CI [0.009-0.512], p=0.009). Complex cytogenetics at the time of initial diagnosis was associated with inferior OS, though statistical significance was not reached (table II).
Conclusion
Our data suggests that upfront TKI therapy is associated improved survival outcomes in pts with MLN-eo and is effective in preventing blastic transformation from chronic phase disease. As proposed, the driver oncogene most likely occurs in hematopoietic stem cells/progenitor cells in this entity. Upfront TKI can potentially suppress, even in some cases eradicate the malignant clone. The study is limited due to small sample size and retrospective nature, and larger study is needed to validate our observation.
Sokol: Kyowa-Kirin: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees. Shah: Novartis: Consultancy, Other: Expenses; Pfizer: Consultancy, Other: Expenses; Amgen: Consultancy; Precision Biosciences: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: Expenses, Research Funding; Pharmacyclics/Janssen: Honoraria, Other: Expenses; Acrotech/Spectrum: Honoraria; BeiGene: Consultancy, Honoraria; Incyte: Research Funding; Jazz Pharmaceuticals: Research Funding; Servier Genetics: Other; Bristol-Myers Squibb/Celgene: Consultancy, Other: Expenses; Adaptive Biotechnologies: Consultancy. Lancet: Agios: Consultancy; AbbVie: Consultancy; Celgene/BMS: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Jazz: Consultancy. Kuykendall: Protagonist: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BluePrint Medicines: Honoraria, Speakers Bureau; Prelude: Research Funding; Abbvie: Honoraria; Incyte: Consultancy; Novartis: Honoraria, Speakers Bureau; PharmaEssentia: Honoraria; Celgene/BMS: Honoraria, Speakers Bureau; CTI Biopharma: Honoraria. Padron: Stemline: Honoraria; BMS: Research Funding; Kura: Research Funding; Blueprint: Honoraria; Incyte: Research Funding; Taiho: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal